Iantrek Raises $42 Million Series C to Launch AlloFlo™ Uveo, a Breakthrough Interventional Glaucoma Therapy
New hope for millions of glaucoma patients as funding advances surgical access to the uveoscleral pathway
WHITE PLAINS, N.Y., Aug. 20, 2025 (GLOBE NEWSWIRE) -- Iantrek, Inc., a pioneer in bio-interventional ophthalmic surgery (BIOS), today announced the close of a $42 million Series C financing round, which will fund the U.S. commercial launch of AlloFlo™ Uveo, a first-of-its-kind surgical solution targeting the uveoscleral pathway, as well as broader pipeline expansion. The Series C was led by U.S. Venture Partners (USVP), joined by aMoon Fund, along with existing investors Visionary Ventures, Sectoral Asset Management, Radius Special Situations Fund, and Civilization Ventures.
“Our mission is to address a major unmet need in glaucoma care, and this fundraise marks a pivotal moment in helping us achieve it,” said Adam Szaronos, CEO of Iantrek. “More than 2.5 million eyes in the U.S. are experiencing waning efficacy from previous Minimally Invasive Glaucoma Surgery (MIGS) procedures targeting the trabecular pathway. AlloFlo™ Uveo enables access to the uveoscleral pathway, the eye’s other natural drainage system—much like giving a thoracic surgeon the ability to enhance the second lung.”
Unlocking the Other Half of the Eye’s Drainage System
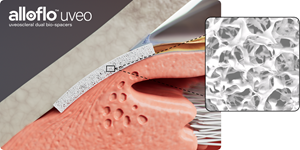
Glaucoma is a chronic, vision-threatening disease that causes irreversible damage to the optic nerve. Glaucoma management generally involves reducing intraocular pressure (IOP) by increasing aqueous drainage or decreasing aqueous production. In healthy eyes, two distinct outflow pathways regulate aqueous drainage: the trabecular and the uveoscleral. While existing MIGS devices focus exclusively on the trabecular outflow pathway, Iantrek’s BIOS platform introduces a breakthrough in minimally invasive access to the uveoscleral pathway—a route long targeted by pharmaceuticals but, until now, lacked technological options to enhance surgically.
“This raise reflects strong confidence in Iantrek’s bold vision and disruptive approach to interventional glaucoma,” said Casey Tansey, General Partner at USVP. “Their platform is redefining what’s possible by combining simplicity, precision, and improved outcomes.”
Clinical Evidence and Early Adoption
In 2025, the American Academy of Ophthalmology published a peer-reviewed study in Ophthalmology Science demonstrating long-term efficacy and safety in patients having undergone bio-reinforced cyclodialysis with AlloFlo Uveo. Key findings after 2 years included a mean reduction of intraocular pressures (IOP) of 34%, use of IOP-lowering medications decreased by >60%, and no serious product-related adverse events reported.1

AlloFlo Uveo - Uveoscleral Dual Bio-Spacers

AlloSert Uveo - Uveoscleral Spacer Delivery Platform
“With approximately 3,000 procedures performed through its early access program, surgeon demand for uveoscleral pathway access is clear,” said Sean Ianchulev, MD, MPH, Professor of Ophthalmology at the New York Eye and Ear of Mount Sinai and Chairman of the Board of Iantrek.
“Iantrek’s bio-interventional approach is currently the only surgical solution targeting this major outflow pathway with the highest therapeutic index in glaucoma treatment.”
Looking Ahead
Iantrek’s portfolio includes AlloFlo™ Uveo, AlloSert™ Uveo, and C.Rex™—next-generation bio- interventional and micro-interventional solutions designed to improve surgical precision, and access to the eye’s natural outflow pathways. AlloFlo™ Uveo is the first allogeneic graft tailored for durable implantation and bio-integration in ophthalmic applications. The company has also completed first-in-human trials for its next bio-interventional product, with commercial launch expected in 2026.
“Iantrek’s sophisticated solution delivers significant clinical value and provides a new minimally invasive option for patients with glaucoma,” said Todd Sone, General Partner at aMoon Fund. “We are looking forward to working with Adam Szaronos, Dr. Sean Ianchulev, and the whole team on their mission to provide next-generation glaucoma treatment to the many thousands of patients in need.”
About Iantrek, Inc.
Founded by ophthalmic innovator Sean Ianchulev, MD, MPH, Iantrek is a venture-funded medical technology company pioneering bio-interventional and micro-interventional products for ophthalmic surgery. The company’s portfolio includes AlloSert™ Uveo, AlloFlo™ Uveo, CanaloFlo™, and C.Rex™— bio-interventional and micro-interventional solutions for the management of glaucoma. For more information, visit iantrekmed.com.
Media Contact:
Amy Phillips
amyphillipspr@gmail.com
412-327-9499
- Bio-interventional Uveoscleral Outflow Enhancement Surgery for Primary Open-Angle Glaucoma: 2-Year Results of Cyclodialysis with Scleral Allograft Reinforcement. Calvo, Ernesto et al. Ophthalmology Science, Volume 5, Issue 4, 100727
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/b27f7d5b-2f61-4dad-a4c6-c508917f2b02
https://www.globenewswire.com/NewsRoom/AttachmentNg/6ce7574d-5cde-4cf7-bcac-eb9031602eab

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.